Age Accelerating “Zombie Cells” – A New Study Sheds Light on These Unique Cells
Senescent cells, or “zombie cells,” are unique in that they ultimately cease multiplying but do not die off as expected.
Researchers have found a new pathway for the buildup of “zombie cells,” which promote aging.
Senescent cells, or cells that have lost their ability to divide, increase with age and are major contributors to age-related illnesses such as cancer, dementia, and cardiovascular disease. In a new study, a team led by the University of Pittsburgh and UPMC Hillman Cancer Center researchers discovered a method through which senescent, or “zombie,” cells develop.

Patricia Opresko, Ph.D., professor of environmental and occupational health and of pharmacology and chemical biology at the University of Pittsburgh and co-leader of the Genome Stability Program at UPMC Hillman Cancer Center. Credit: Patricia Opresko
The study, which was recently published in the journal Nature Structural & Molecular Biology, demonstrates for the first time that oxidative damage to telomeres — the protecting tips of chromosomes that behave like plastic caps at the end of a shoelace — can cause cellular senescence. These discoveries might ultimately result in new treatments that promote healthy aging or fight cancer.
“Zombie cells are still alive, but they can’t divide, so they don’t help replenish tissues,” said senior author Patricia Opresko, Ph.D., professor of environmental and occupational health and of pharmacology and chemical biology at Pitt. “Although zombie cells don’t function properly, they’re not couch potatoes — they actively secrete chemicals that promote inflammation and damage neighboring cells. Our study helps answer two big questions: How do senescent cells accumulate with age, and how do telomeres contribute to that?”
When a healthy human cell divides to create two identical cells, a little bit of DNA is shaved off the tip of each chromosome, causing telomeres to get shorter with each division. However, it is unknown if a cell may divide so often in a person’s lifetime that its telomeres fully degrade, resulting in a zombie-like condition. For decades, scientists have known that telomere shortening causes senescence in lab-grown cells, but they could only assume that DNA damage at telomeres could convert cells into zombies.
This hypothesis could not previously be tested since the techniques used to damage DNA were non-specific, creating lesions across the entire chromosome.
“Our new tool is like a molecular sniper,” explained first author Ryan Barnes, Ph.D., a postdoctoral fellow in Opresko’s lab. “It creates oxidative damage exclusively at the telomeres.”
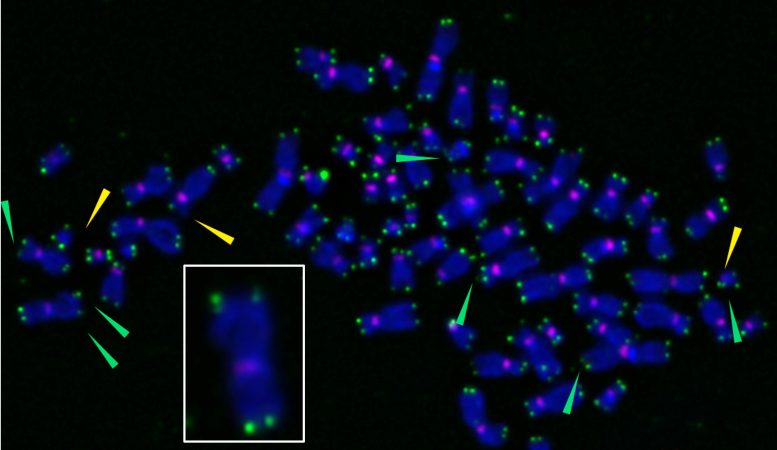
X-shaped chromosomes are stained purple, and telomeres appear as green spots at chromosome tips. When researchers used a novel tool to induce oxidative damage specifically at telomeres, they can become fragile (green arrows), sending cells into senescence. The inset shows an enlarged chromosome with fragile telomeres, indicated by multiple green spots at chromosome tips. Credit: Barnes et al., Nature Structural & Molecular Biology, (2022)
To develop such marksman-like precision, the team used a special protein that binds exclusively to telomeres. This protein acts like a catcher’s mitt, grabbing hold of light-sensitive dye “baseballs” that the researchers tossed into the cell. When activated with light, the dye produces DNA-damaging reactive oxygen molecules. Because the dye-catching protein binds only to telomeres, the tool creates DNA lesions specifically at chromosome tips.
Using human cells grown in a dish, the researchers found that damage at telomeres sent the cells into a zombie state after just four days — much faster than the weeks or months of repeated cell divisions that it takes to induce senescence by telomere shortening in the lab.
“We found a new mechanism for inducing senescent cells that is completely dependent on telomeres,” explained Opresko, who also co-leads the Genome Stability Program at UPMC Hillman. “These findings also solve the puzzle of why dysfunctional telomeres are not always shorter than functional ones.”
Sunlight, alcohol, smoking, poor diet, and other factors generate reactive oxygen molecules that damage DNA. Cells have repair pathways to patch up DNA lesions, but, according to Opresko, telomeres are “exquisitely sensitive” to oxidative damage. The researchers found that damage at telomeres disrupted DNA replication and induced stress signaling pathways that led to senescence.
“Now that we understand this mechanism, we can start to test interventions to prevent senescence,” said Barnes. “For example, maybe there are ways to target antioxidants to the telomeres to protect them from oxidative damage.”
The findings could also inform the development of new drugs called senolytics that home in on zombie cells and kill them.
“By reducing the accumulation of zombie cells, which contribute to degenerative diseases, we might be able to promote ‘healthspan’ — the length of time that a person is healthy,” he added.
Reference: “Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening” by Ryan P. Barnes, Mariarosaria de Rosa, Sanjana A. Thosar, Ariana C. Detwiler, Vera Roginskaya, Bennett Van Houten, Marcel P. Bruchez, Jacob Stewart-Ornstein, and Patricia L. Opresko, 30 June 2022, Nature Structural & Molecular Biology.